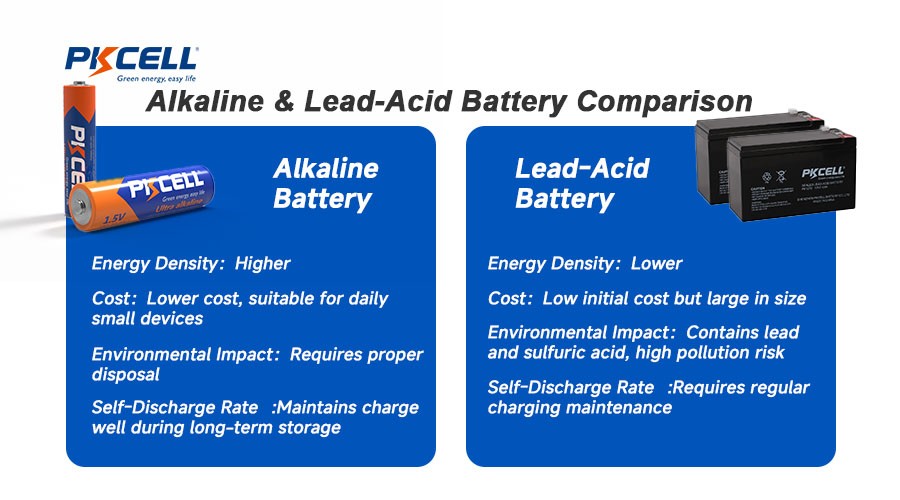
Key Highlights
- Alkaline batteries typically use a zinc and manganese dioxide reaction, while lead acid batteries consist of lead dioxide plates and sulfuric acid electrolyte.
- Alkaline batteries generally offer a higher energy density compared to lead acid batteries. They are lighter and more efficient for portable devices.
- Lead acid batteries excel in high discharge applications. It delivers substantial current over short periods, which makes them ideal for vehicles and heavy machinery.
Introduction
Many people use lead-acid and alkaline batteries. These two types of batteries have distinct characteristics that make them suitable for different applications. Understanding their strengths and weaknesses can help you make informed choices based on their specific needs. In this comparison, we will dive deeper into the unique features of alkaline and lead acid batteries.
Understanding Alkaline Batteries
Alkaline batteries are the kind that many people use every day. You can get them in different sizes. A lot of people put them in remote controls, and others use them in flashlights. These batteries rely on a chemical reaction between zinc and manganese dioxide to generate power. Their design enables them to offer a longer shelf life and greater efficiency than many alternatives, making them a popular choice for everyday electronic needs.
The Chemistry Behind Alkaline Batteries
Alkaline batteries are called that because they use an alkaline solution, which contains an alkali metal salt known as potassium hydroxide. This solution is called potassium hydroxide. The alkaline solution is the electrolyte in these batteries. It lets ions move from one side to the other. This is how the electrical current can flow inside alkaline batteries. When the battery is in use, the zinc anode gives up some electrons. The zinc then turns into ions. These electrons go out, move through a wire, and reach the manganese dioxide cathode. The cathode takes in the electrons. Next, the manganese dioxide reacts with the alkaline solution. This makes something new called manganese oxide hydroxide. The way ions and electrons move gives power to our devices.
Common Uses and Applications
Alkaline batteries are used by many people in a lot of different electronics. They work well and give a steady power supply. Here are some common uses for alkaline batteries:
- Remote controls: People often use alkaline batteries in the remote controls for their TVs and audio systems.
- Consumer electronics: A lot of small gadgets, like clocks, radios, calculators, and cordless phones, get their power from alkaline batteries.
- Toys and Games: You will find alkaline batteries in many toys that need batteries and in small handheld games.
Alkaline batteries are in many homes. They are easy to get and use. A lot of people like alkaline batteries because the price is good and the shelf life is long.

Advantages of Alkaline Batteries
- One good thing about alkaline batteries is their long shelf life. They can keep their charge for a long time, even when you are not using them.
- Alkaline batteries are cheap, and you can buy them at almost any store. This is part of the reason why people use alkaline batteries for so many things.
- Another benefit of alkaline batteries is that they give out a steady discharge rate. Most of the time, they keep the voltage the same when you use them. This is good for the things that need a stable power supply to work well.
Disadvantages of Alkaline Batteries
- A big disadvantage of Alkaline Batteries is that they might leak. This tends to happen with old batteries or if you keep them in places that are too hot or too cold.
- These batteries have heavy metals in them, like zinc and other stuff. You should not throw them out with your normal trash at home. It is best to use a safe disposal plan, like local recycling programs, for alkaline batteries.
- Another thing you need to know is that alkaline batteries do not stay good as long on the shelf as some other batteries like lithium ones. The shelf life of alkaline batteries is okay for most things that people use, but they do have a longer life compared to some alternatives, so you should always check the date on them.
Exploring Lead Acid Batteries
Lead-acid batteries started to be used in the 19th century. These rechargeable batteries are strong, and people know they last a long time. They are also not expensive. That is why people use them for many things. You can find them in cars, and they are used to store energy from renewable sources. Unlike alkaline batteries, which you use just once, acid batteries can be recharged. This is what makes acid batteries different.
How Lead Acid Batteries Work
A lead-acid battery runs because there is a chemical reaction between the lead plates and the sulfuric acid. When you use this battery, lead sulfate begins to form on both plates, the positive one and the negative one. The sulfuric acid in the electrolyte will also get weaker as this reaction goes on.
The positive plate is usually made of lead dioxide and antimony. It reacts with dilute sulfuric acid and makes lead sulfate. When this happens, it gives out some electrons. The negative plate is made of metallic lead. It also forms lead sulfate but takes in the electrons. The electrons move from the negative plate to the positive plate. They travel on an outside path and this is what makes the electric current.
Applications of Lead Acid Battery
Lead-acid batteries are known for giving a lot of power when you need them. People trust these acid batteries because they work well and are not too expensive. Here are some key applications:
- Car batteries: Lead-acid batteries are the most-used car batteries. They are needed because they provide a high current to start the engine.
- Uninterruptible Power Supplies (UPS): Acid batteries work as backup in uninterruptible power supplies. When there is a power cut, they help computers, servers, and other important devices stay on.
- Emergency Lighting: You may see these batteries in emergency lighting. When the main power stops, acid batteries give light. They last a long time and give a strong current.
Lead-acid batteries are important in our lives. You can see them in many things that we use every day.

Benefits of Using Lead Acid Batteries
- Lead-acid batteries are used a lot because they are reliable and do not cost much. They are one of the cheapest types of batteries you can get, especially when you need a big one.
- These batteries are small and not heavy. They have a high energy density. This lets them hold a lot of energy. They are good to use when there is not much space.
- You can also recycle lead-acid batteries. The lead in these acid batteries can be used again to make new batteries. This helps make the disposal of them better for the environment.
Limitations of Lead Acid Batteries
- A big issue is that if you let them run out of power too much when using a charger, they can get damaged. They will not last as long as before. The batteries also will not hold as much power as they did at first.
- Also, acid batteries do not last as long as lithium ones. The service life of acid batteries can go down if the temperature changes. The way you charge them can also lower their service life.
- Another problem is that lead is a heavy metal in acid batteries. If people do not get rid of acid batteries in the right way, it can hurt the environment. It is very important to recycle these batteries in a safe way.
Difference Between Lead Acid and Alkaline Battery
|
Feature |
Lead Acid Battery |
|
|
Rechargeability |
Not rechargeable |
Rechargeable |
|
Voltage |
1.5V per cell |
2V per cell |
|
Energy Density |
Higher |
Lower |
|
Cost |
Lower |
Higher |
|
Applications |
Low-drain device: remote control, toys |
High-power devices: Car, Ebike, forklift |
|
Electrolyte |
potassium hydroxide |
Uses lead plates and sulfuric acid |
Alkaline batteries have a long shelf life. They also cost less. You will see they are good for things like remote controls and clocks. These items do not need much power to work. If you need power for big tasks, like starting car engines or running UPS systems, acid batteries will be better for you.
Energy Density Comparison
When you look at energy density, you can see that lead-acid batteries have more energy storage capacity than alkaline batteries. A lead-acid battery will have and store more energy than an alkaline battery that is the same size or weight. This is one big reason why people use them in portable electronics and electric vehicles. These batteries are lighter, and they do not take up much space. That makes them a good fit for many of these devices.
Lifespan and Durability
Alkaline batteries have a longer shelf life than lead batteries do. They keep their charge for a longer time even if you do not use them for a while. This is good for things like remote controls or smoke detectors that you do not always need.
Acid batteries, like deep-cycle acid batteries, are made to be used many times for deep-discharge applications. You can charge and discharge them again and again. This is good if you want to use them for solar power storage. They also work well in electric vehicles.
Environmental Impact and Recycling
It is good to think about how batteries and their terminals can hurt the world we live in. Both alkaline and acid batteries have heavy metals in them. They can be worse for the environment.
Lead can hurt people and the environment. If you throw away acid batteries the wrong way, the lead from them gets into the ground and water. This can cause problems for us and other living things. When we recycle them, the lead can be used again in new acid batteries.
Alkaline batteries might seem better than acid batteries, but you still need to throw them away the right way. A lot of places will let you recycle alkaline batteries. You should check what your town asks you to do.
Cost Analysis and Accessibility
Alkaline batteries are cheaper than acid batteries and can be easily found in many places. Because of this, many people choose them for a lot of different uses. Lead-acid batteries can help you save money over time. It is easy to charge them again. The choice between acid batteries and Alkaline batteries depends on what you want and how much you plan to spend.
Initial Investment and Long-term Savings
Alkaline batteries have a lower price when you buy them at first. The cost makes it easy for people to get alkaline batteries for their daily use. However, when you think about costs over a long time, you need to look at more than just the price you pay first. Lead acid batteries and AGM batteries might seem like they are priced higher at the start, but their low cost over time will help you save money. You can keep using and charging these acid batteries and AGM batteries many times. So, you pay more first, but you will get that money back as time goes on. The first payment is not a problem, because you keep making use of it for a long time. Both AGM batteries and acid batteries work well, last a long time, and give good value for your money.
When to Choose an Alkaline Battery
Alkaline batteries work well in things that do not use a lot of power. They come with a long shelf life. This means that you can keep them for a long time before they run out. This is very good for devices like remote controls, clocks, and smoke detectors. If you want a battery that will keep going, alkaline batteries are a good choice.
Alkaline batteries are cheap, and they can be a good option for many people. You do not have to change them often, and that is one thing many of us look for. They are easy to find, and they do the job for daily use. If you want a power source you can trust, alkaline batteries are a good choice.
Ideal Scenarios for Lead Acid Battery
Lead-acid batteries are used when you need a lot of power. People trust these acid batteries for their strength. You will find that most car batteries are the acid type. These batteries give the power you need to get your car started. Car batteries are made to be tough. They work well, even when it is very hot.
Another main use for acid batteries is in uninterruptible power supplies (UPS). These systems need acid batteries to take over and give power if the main power goes out. This makes sure that important things like computers, servers, and medical equipment keep running the right way.
Lead-acid batteries, also called acid batteries, are used in emergency lighting systems. They can work for a long time and help make sure there is steady power. During an emergency, these acid batteries help people feel safe and get the power they need.
Why Choose PKCELL Batteries
PKCELL offers many high-quality batteries to meet the power needs of people. This is good if you want something that will work for a long time and you need it to be reliable. PKCELL provides both alkaline and acid batteries, so it’s easy to choose the one that fits you best. Our alkaline batteries have a long shelf life. You can keep these batteries for a long time, and they will still work when you need them. At PKCELL, we want you to get the most from every battery you use. Choose PKCELL for both your acid batteries and alkaline batteries. You will see the good quality and better performance each time you use them.
Conclusion
To sum up, it is good to know what makes alkaline batteries different from acid batteries. Knowing this can help you pick the one that fits your needs. Alkaline batteries are good for things you use every day. They are simple to use. Acid batteries, like lead-acid batteries, work well in cars and in cases where you want something to last a long time and handle daily use.
When you choose between these batteries, think about the energy density, how long each battery will last, the cost, and what they might do to the environment. PKCELL batteries can give you power you can trust. Make the right choice to get good and lasting energy for all your devices.
Frequently Asked Questions
What is the Key Difference Between Alkaline and Lead Acid Batteries?
Alkaline batteries use potassium hydroxide for the electrolyte. Lead-acid batteries use sulfuric acid as the electrolyte instead, and some lead-acid batteries may contain an alloy of lead. Lead-acid batteries often have more energy density than alkaline batteries. Because of this, acid batteries are better for things that use a lot of current at one time.
Which type of battery is more environmentally friendly: alkaline or lead-acid?
Lead-acid batteries are bad for the environment because they have lead in them. Lead is one of the heavy metals found in acid batteries and other batteries too. That is why it is important to recycle them. This helps with proper disposal and cuts down on harm to the environment.

 USB Rechargeable Lithium Battery
USB Rechargeable Lithium Battery